If you would like to talk to someone about epilepsy, our trained advisers are here to help.
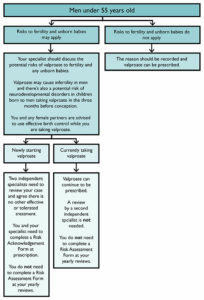
 Men currently taking sodium valproate will not need sign off from two independent specialists to continue their treatment, the Medicines and Healthcare products Regulatory Agency has confirmed today.
Men currently taking sodium valproate will not need sign off from two independent specialists to continue their treatment, the Medicines and Healthcare products Regulatory Agency has confirmed today.
The update means they will also not need to sign a Risk Acknowledgement Form.
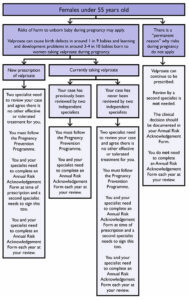
However, any new valproate prescriptions for men or for women under the age of 55 will still need two specialists to independently agree that “there is no other effective or tolerated treatment, or there are compelling reasons that the reproductive risks do not apply”, the MHRA said.
After a new prescription of valproate, women will then need to sign an Annual Risk Acknowledgement Form, while men will only need to sign a Risk Acknowledgement Form once.
These changes to the prescription guidance were introduced in January 2024, affecting men and boys for the first time. The MHRA has been tightening prescription rules around valproate for women since 2018. This followed decades of problems in babies born to mothers taking valproate without women being made aware of the risks, which became a “medical scandal”.
According to the MHRA, there is a risk that about one in nine babies born to mothers taking valproate will have birth defects and about 30-40 of 100 will have learning difficulties.
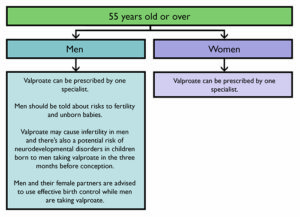
In men taking valproate, the organsiation warns that there is a risk of reduced fertility.
However, no one should stop taking their medication without speaking to their doctor first, as this could result in more or worse seizures.
The MHRA has been advised on this guidance by the Commission on Human Medicines (CHM). The CHM is a public body responsible for advising on the safety, efficacy and quality of medical products.
According to the guidance, women and girls under the age of 55 currently taking valproate and who have not yet been reviewed by two independent specialists, will need a second specialist to sign off on the prescription of valproate at their next annual review.
Future annual reviews will then only require one specialist.
Specialists able to review the use of valproate, according to the MHRA, include consultant neurologists (adult and paediatric), specialist nurses in relevant disciplines and specialist pharmacists. The MHRA has posted a full list on its website.
Men currently taking valproate are advised to use effective contraception and not to donate sperm while taking valproate and three months after stopping. The MHRA says they should speak to a health professional if they are planning to have a baby.
If you have any questions or concerns, you can speak to the Epilepsy Action Helpline by calling 0808 8005050, emailing or starting a live chat with us.
 Visit our website for more information on sodium valproate risks and rules.
Visit our website for more information on sodium valproate risks and rules.
Here to support you
Send us your question
Send a question to our trained epilepsy advisers. (We aim to reply within two working days).




